Overview: This blog reviews new electrochemical energy sources for EV/HEV applications. The features, advantages, and disadvantages of each energy source are also discussed.
Significant interest has been shown in electro-mobility applications, especially electric vehicles, and hybrid electric vehicles. Electric and hybrid electric car sales are expected to surpass those of gasoline and diesel vehicles by 2040. The purpose of this study is to provide a critical overview of electrochemical energy being developed for EV/HEV use.
Electrochemical Energy Sources
Electric motor drives and other ancillary components in EVs/HEVs, such as HVAC, lighting, and AV systems, can all be powered by electrochemical energy sources. Currently available electrochemical energy sources can only produce either a high specific energy output or a high specific power output, but not both. Batteries, fuel cells, and ultracapacitors are only three of the many electrochemical energy sources currently on the market.
Batteries
A battery is a device that can transform electrochemical energy from active materials into electrical energy. A conventional battery has three main components: the positive electrode, the negative electrode, and the electrolyte. A lead-acid battery, a nickel-based battery, or a lithium-based battery are the three most common options for EV/HEV applications.
Some of the earliest EV/HEV applications made use of lead-acid batteries, and these batteries were made commercially available early on. The positive electrode in a standard lead-acid battery is made from lead dioxide, whereas the negative electrode is made from metallic lead. Improved lead-acid batteries, also known as value-regulated lead-acid (VRLA) batteries, use two different types of electrolyte—absorbed electrolyte and gelled electrolyte—instead of just one. When compared to standard batteries, VRLA batteries' bi-shaped design significantly lowers evaporation, leakage, and vibration.
-
Nickel-Based Batteries
Numerous types of nickel-based batteries, including nickel-iron (Ni-Fe), nickel-cadmium (Ni-Cd), nickel-zinc (Ni-Zn), and nickel-metal hydride (Ni-MH) batteries, are used in a variety of applications, including EVs and HEVs. The Ni-MH battery, which uses potassium hydroxide solution as the electrolyte and nickel hydroxide as both the positive and negative electrodes, is the most widely used battery in EVs and HEVs.
-
Lithium-Ion Based Batteries
Issues with lithium metal storage, transport, and use are among the most serious in the industry. Thus, the lithium-ion (Li-ion) battery is formed by substituting an intercalation material for the lithium metal that would otherwise serve as the negative electrode. The positive and negative electrodes of a typical Li-ion battery are composed of lithium intercalation compounds. Lithium ions are added to and taken away from the battery's positive and negative electrodes throughout the charging and recharging procedures. In theory, the migration of lithium ions results in no net change.
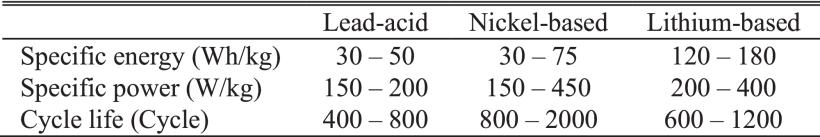
Table 1. Comparisons of Typical Batteries. Source: Lee et al.
Specific energy, specific power, and cycle life are three important key indices typically used to assess battery performance. A battery's specific energy is its total potential energy, whereas its specific power is the amount of energy it actually delivers over a given time period. The cycle life of a battery is defined as the number of times it may be charged and discharged before showing any noticeable deterioration. Table 1 provides a summary of the main differences and similarities between common battery types.
Fuel Cells
A fuel cell is a type of electrochemical energy source that can transform chemical energy into electrical energy. Alkaline fuel cell (AFC), phosphoric acid fuel cell (PAFC), proton exchange membrane fuel (PEMFC), and direct methanol fuel cells are four viable possibilities for EV/HEV applications (DMFC).
-
Alkaline Fuel Cell (AFC)
Using only hydrogen, oxygen, and a recirculating electrolyte, AFCs have been widely hailed as the first truly viable fuel cell. Its excellent efficiency, developed technology, and low cost are undeniable benefits. The matrix is saturated with an alkaline solution, separating two porous electrodes. Potassium hydroxide (KOH) is often used because of its high heat-transfer efficiency in AFC. For AFC to function, a redox reaction must take place.
-
Phosphoric Acid Fuel Cell (PAFC)
PAFC is widely regarded as the first commercialized fuel cell in the electric power industry. It utilizes liquid phosphoric acid as its electrolyte, while platinum-coated carbon is a catalyst. Electrons can move from the negative electrode to the positive electrode via an external circuit, while positively-charged hydrogen ions are able to transfer from the positive electrode to the negative electrode via an acidic electrolyte. Since PAFC needs to operate in a relatively higher temperature range, phosphoric acid is usually employed as an electrolyte. When PAFC generates electricity, it produces water as a by-product.
-
Proton Exchange Membrane Fuel Cell (PEMFC)
A proton exchange membrane fuel cell (PEMFC), also known as an ion exchange membrane fuel cell or a solid polymer electrolyte fuel cell, uses a proton conducting membrane as its electrolyte. The PEMFC was the first fuel cell used by NASA for the Gemini space program in the 1960s, and it is famous for its high power density. In order to prolong the membrane's integrity and service life, this fuel cell type is often operated at a low temperature which necessitates the use of a costly platinum metal catalyst for chemical processes. Just like PAFC, PEMFC generates water as a byproduct of its electric power generation.
-
Direct Methanol Fuel Cell (DMFC)
Direct methanol fuel cells (DMFC) are advantageous due to their use of fuel that is both readily available and relatively easy to store. In the meantime, methanol can be indirectly converted as a hydrogen reformate gas combination and stored within the fuel cell (IMFC). As with other fuel cells, IMFC operates most effectively when supplied with hydrogen. The DMFC's ability to generate carbon dioxide and water is dependent on its design.
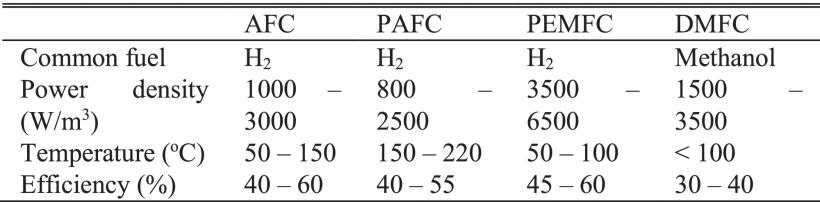
Table 2. Comparisons of Typical Fuel Cells. Source: Lee et al.
Four basic critical indices—common fuel, power density, temperature, and efficiency—are employed to assess the functioning of fuel cells. Temperature is the operating temperature, whereas power density is the total power produced by a full cell per unit of volume. Table 2 lists the comparisons between typical complete cells.
Ultracapacitors
A capacitor is a basic device that stores and releases electrons by means of a dielectric between two metallic electrodes. There are two primary varieties of classical capacitors; electrostatic capacitors and electrolytic capacitors. Unlike the latter, the former does not rely on electrolytic materials. As of right now, none has sufficient capacity for EV/HEV uses. One hopeful answer to this problem is the ultracapacitor, a relatively new technology that comes in two main varieties: the double-layer capacitor and the pseudocapacitor.
-
Double Layer Capacitor
To store charge with the electrode surface and the electrolyte separated, the concept of the two-layer capacitor was developed. Using the latest in nanotechnology, highly porous materials can be created for use as capacitor electrodes. The high capacitance of the two-layer capacitor is made possible by this novel material property. It's important to keep in mind that the charging and discharging processes of a double-layer capacitor do not involve any chemical reactions; hence the operating principle of such a device is said to be non-Faradic.
-
Pseudocapacitor
For a pseudocapacitor, the charging and discharging processes are chemical reactions known as the Faradic process, and the oxidation-reduction events that take place at the interface set it apart. Pseudocapacitors, which utilize the Faradic technique, typically provide greater capacitance and energy density than double-layer capacitors.
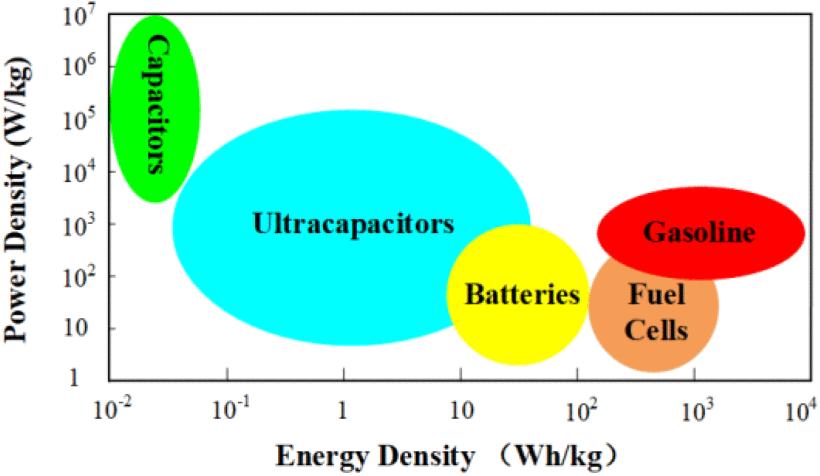
Fig. 1. Comparisons among various energy sources for EVs/HEVs. Source: Lee et al.
Fig. 1 shows how batteries, fuel cells, capacitors and ultracapacitors, and gasoline, four of the most important electrochemical energy sources, compare to each other.
Summarizing with key points:
Some of the takeaways from the article are as follows:
- Several different types of electrochemical energy sources are commercially available today, including batteries, fuel cells, and ultracapacitors.
- Most EV/HEVs use either lead-acid batteries, nickel-based batteries, or lithium-based batteries.
- It is common practice to evaluate a battery's worth by measuring its specific energy, specific power, and cycle life.
- Fuel cells are an electrochemical energy source that converts chemical energy into electrical energy.
- Four feasible options for EV/HEV applications are the alkaline fuel cell (AFC), phosphoric acid fuel cell (PAFC), proton exchange membrane fuel (PEMFC), and direct methanol fuel cell (DMFC).
- An ultracapacitor is a type of modern capacitor that can be either a double-layer or a pseudocapacitor.
- The idea of a two-layer capacitor was created to store charge with the electrode surface and the electrolyte physically separated.
- Pseudocapacitors, which employ the Faradic method, are often superior to double-layer capacitors in terms of capacitance and energy density.
This blog post is part of a full research article that has been published in IEEE Access.







